October 2024 TPJ Editor choice
Highlighted publication:
Wheat domestication alters root metabolic functions to drive the assembly of endophytic bacteria
https://doi.org/10.1111/tpj.16972
Lost in domestication: has modern wheat left its microbial allies behind?
The domestication of wheat (Triticum aestivum), which began approximately 10,000 years ago in the Fertile Crescent, was a pivotal event in the first agricultural revolution. It marked the shift from a hunter-gatherer lifestyle to one of settlement and agriculture. A key milestone in this process was the domestication of wild emmer wheat, which gave rise to cultivated tetraploid durum wheat and represents an important stepping stone towards modern bread wheat, which emerged 1,500-2,000 years later and is the most widely grown type of wheat today. Domestication brought about substantial changes in wheat’s morphology and development, including altered flowering time, larger grains, increased yield, reduced seed dormancy and the elimination of seed shattering.
Changes caused by domestication are not restricted to the crop, but also affect organisms interacting with it. Microorganisms living on and within the plant, collectively referred to as the plant’s microbiome, play a crucial role for plant fitness, influencing growth, resistance and resilience throughout the plant’s life cycle. Roots are a major interface between plants and soil microbes, with many microbes living as endophytes within the roots or colonising the surrounding soil (rhizosphere). Host plants release exudates to attract beneficial rhizospheric and endophytic bacteria, and the composition of these exudates shapes the root microbiome. While several studies found that domestication reduced the diversity of rhizospheric microbes, little research has explored how domestication affected the diversity of endophytes.
Deng and colleagues chose three wild emmer accessions and three domesticated elite cultivars that represent six distinct branches of the wheat phylogenetic tree for their analyses of the endophyte microbiome. DNA was extracted from roots of these six varieties grown in the same soil and bacterial taxa were identified by sequencing prokaryote-specific 16S rRNA genes. Alpha diversity indices, which describe the diversity in microbial species and their abundance, were significantly higher for the domesticated cultivars, suggesting that domestication has increased endophyte diversity. While 110 bacterial genera were detected in both groups, albeit with differences in abundance, only one genus, Serratia, was unique to wild varieties and none were exclusive to domesticated cultivars. Serratia is known to enhance plant growth, particularly under salt stress and nutrient deprivation and may be an important factor for wild wheat’s stress resilience.
Based on significant pairwise correlations, the authors constructed bacterial interaction networks for both wheat groups (Fig. 1A). Network connectivity (i.e., the number of co-occurrences) was higher for wild than domesticated wheat; domestication thus seems to have destabilised wheat’s bacterial co-occurrence network. The analysis also identified several genera, including growth-promoting Chryseobacterium, Massilia and Lechevalieria, as keystone taxa that play central roles in these bacterial networks.
In parallel with their microbiome analysis, the authors generated metabolic profiles of root exudates from the six wheat varieties. Eight and six metabolites were found exclusively in wild and domesticated wheat, respectively, indicating that domestication substantially altered root metabolism. The researchers integrated these metabolomics data with their microbiome data and detected significant correlations between keystone taxa and multiple metabolites. Among these, the amino acid L-tyrosine stood out for its higher abundance in wild wheat roots and its strong correlation with the presence of Chryseobacterium. To explore this further, the team grew wheat plants in controlled conditions and exposed them to Chryseobacterium, L-tyrosine or a combination of both. While neither treatment altered shoot or root growth of domesticated wheat varieties, the combined exposure to L-tyrosine and Chryseobacterium reduced root elongation, but increased root fresh weight, of wild wheat (Fig 1B). Several Chryseobacterium species are known to promote plant growth by solubilising nutrients and producing plant hormones. The observations by Deng et al. support the hypothesis that wild wheat recruits or stimulates Chryseobacterium by the release of L-tyrosine, and that this beneficial relationship was lost during domestication.
Reshaping the microbiome represents a promising strategy for improving crop yield and resilience. Understanding how crops interact with their microbiomes, and how these interactions have changed through domestication, represents an important step towards this goal. The findings by Deng et al. highlight the potential of wild wheats and their microbiomes as valuable resources for improving modern wheat varieties. Bacteria such as Serratia and Chryseobacterium represent exciting candidates in this context, but changes to wheat’s genetic make-up may be required to fully unlock the microbes’ potential.
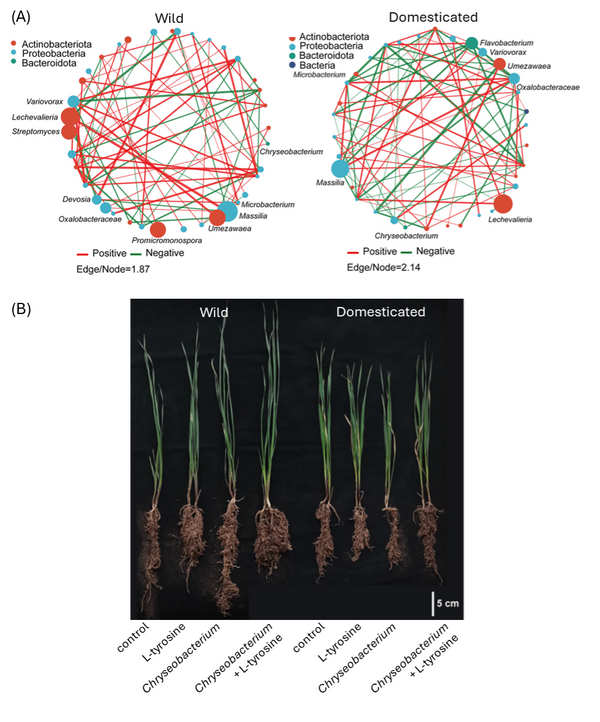
Figure 1. Bacterial root endophytes of wild and domesticated wheat varieties and their effect on plant growth
- Co-occurrence networks of bacterial endophytes in wild (left) and domesticated (right) wheat cultivars. Node size indicates the relative abundance of each taxon. Green lines represent negative correlations and red lines represent positive correlations.
- Phenotype of wild (left) and domesticated (right) wheat plants when exposed to L-tyrosine, Chryseobacterium, or a combination of both.
Figure modified from Deng et al.